Medicinal chemistry perspectives of 1,2,3,4-tetrahydroisoquinoline analogs – biological activities and SAR studies
Abstract
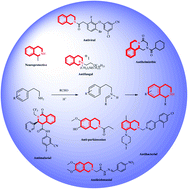
Isoquinoline alkaloids are a large group of natural products in which 1,2,3,4-tetrahydroisoquinolines (THIQ) form an important class. THIQ based natural and synthetic compounds exert diverse biological activities against various infective pathogens and neurodegenerative disorders. Due to these reasons, the THIQ heterocyclic scaffold has garnered a lot of attention in the scientific community which has resulted in the development of novel THIQ analogs with potent biological activity. The present review provides a much-needed update on the biological potential of THIQ analogs, their structural–activity relationship (SAR), and their mechanism of action. In addition, a note on commonly used synthetic strategies for constructing the core scaffold has also been discussed.
- This article is part of the themed collection: 2021 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...