Synthesis and antioxidant activities of berberine 9-O-benzoic acid derivatives†
Abstract
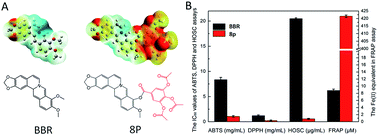
Although berberine (BBR) shows antioxidant activity, its activity is limited. We synthesized 9-O-benzoic acid berberine derivatives, and their antioxidant activities were screened via ABTS, DPPH, HOSC and FRAP assays. The para-position was modified with halogen elements on the benzoic acid ring, which led to an enhanced antioxidant activity and the substituent on the ortho-position was found to be better than the meta-position. Compounds 8p, 8c, 8d, 8i, 8j, 8l, and especially 8p showed significantly higher antioxidant activities, which could be attributed to the electronic donating groups. All the berberine derivatives possessed proper lipophilicities. In conclusion, compound 8p is a promising antioxidant candidate with remarkable elevated antioxidant activity and moderate lipophilicity.


 Please wait while we load your content...
Please wait while we load your content...