Preparation of 1,2-substituted benzimidazoles via a copper-catalyzed three component coupling reaction†
Abstract
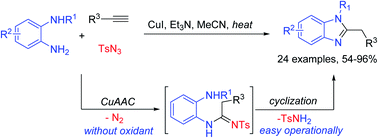
1,2-Substituted benzimidazoles were prepared by simply stirring a mixture of copper catalysts, N-substituted o-phenylenediamines, sulfonyl azides and terminal alkynes. Particularly, the intermediate N-sulfonylketenimine occurred with two nucleophilic addition and the sulfonyl group was eliminated via cyclization. In a way, sulfonyl azides and copper catalysts activated the terminal alkynes to synthesize benzimidazoles.


 Please wait while we load your content...
Please wait while we load your content...