Highly sensitive naphthalimide based Schiff base for the fluorimetric detection of Fe3+†
Abstract
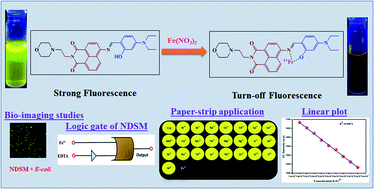
A simple 1,8-naphthalimide based Schiff base probe (E)-6-((4-(diethylamino)-2-hydroxybenzylidene)amino)-2-(2-morpholinoethyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (NDSM) has been designed and synthesized for the specific detection of Fe3+ based on a fluorimetric mode. The absorbance of NDSM at 360 nm increased significantly in acetonitrile : water (7 : 3, v/v) medium only in the presence of Fe3+ ions with a visible colour change from yellow to golden yellow. Likewise, fluorescence emission intensity at 531 nm was almost wholly quenched in the presence of Fe3+. However, other competitive ions influenced insignificantly or did not affect the optical properties of NDSM. Lysosome targetability was expected from NDSM due to the installation of a basic morpholine unit. The LOD was found to be 0.8 μM with a response time of seconds. The fluorescence reversibility of NDSM + Fe3+ was established with complexing agent EDTA. Fe3+ influences the optical properties of NDSM by complexing with it, which blocks C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization in addition to the ICT mechanism. The real-time application of Fe3+ was demonstrated in test paper-based detection, by the construction of a molecular logic gate, quantification of Fe3+ in water samples and fluorescence imaging of Fe3+.
N isomerization in addition to the ICT mechanism. The real-time application of Fe3+ was demonstrated in test paper-based detection, by the construction of a molecular logic gate, quantification of Fe3+ in water samples and fluorescence imaging of Fe3+.


 Please wait while we load your content...
Please wait while we load your content...