Additive-free photo-mediated oxidative cyclization of pyridinium acylhydrazones to 1,3,4-oxadiazoles: solid-state conversion in a microporous organic polymer and supramolecular energy-level engineering†
Abstract
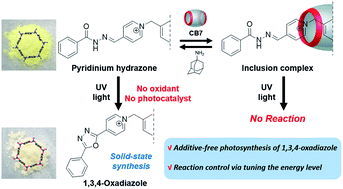
We discovered the efficient catalyst-free, photo-mediated oxidative cyclization reaction of bis-p-pyridinium benzoyl hydrazone (BH1) to 2-pyridinium-5-phenyl-1,3,4-oxadiazoles. This photoreaction is remarkable because it does not require additives (e.g., bases, strong oxidants, or photocatalysts), which are essential in previous reports, and proceeds very effectively even with solid-state microporous organic polymers. Interestingly, we found that the inclusion complexation of BH1 with cucurbit[7]uril (CB7) interferes with the photo-induced electron transfer from BH1 to molecular oxygen through modification of the LUMO energy level, thus inhibiting the photo-medicated oxidative cyclization.


 Please wait while we load your content...
Please wait while we load your content...