A simplified approach for the metal-free polymerization of propylene oxide†
Abstract
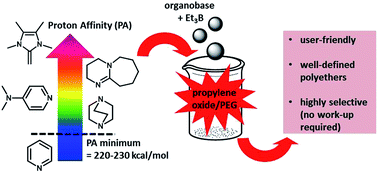
Triethyl borane (Et3B), in combination with phosphazene-type superbases, has recently emerged as a powerful co-catalyst for the anionic polymerization of epoxides. Here, it is demonstrated that the monomer-activating property of Et3B can also compensate for the application of much gentler organobases. This not only results in simpler setups, but also significantly reduces nucleophilicity/basicity-derived side reactions. Notably, this principle applies to such a degree that simple 4-dimethylaminopyridine (DMAP) or 1,4-diazabicyclo[2.2.2]octane (DABCO) can serve to polymerize propylene oxide (PO). With suitable initiators, this results for example in very well-defined block copolyethers (ÐM ≤ 1.03) without requiring work-up to remove side products such as PPO homopolymer. Performance correlates nicely with the corresponding organobase proton affinities (PAs), and a limiting PA of 220–230 kcal mol−1 was identified for successful PO polymerization.


 Please wait while we load your content...
Please wait while we load your content...