Efficiency of liquid tin(ii) n-alkoxide initiators in the ring-opening polymerization of l-lactide: kinetic studies by non-isothermal differential scanning calorimetry†
Abstract
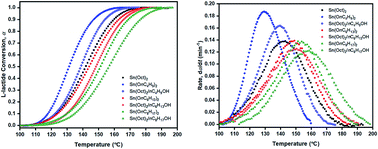
Novel soluble liquid tin(II) n-butoxide (Sn(OnC4H9)2), tin(II) n-hexoxide (Sn(OnC6H13)2), and tin(II) n-octoxide (Sn(OnC8H17)2) initiators were synthesized for use as coordination–insertion initiators in the bulk ring-opening polymerization (ROP) of L-lactide (LLA). In order to compare their efficiencies with the more commonly used tin(II) 2-ethylhexanoate (stannous octoate, Sn(Oct)2) and conventional tin(II) octoate/n-alcohol (SnOct2/nROH) initiating systems, kinetic parameters derived from monomer conversion data were obtained from non-isothermal differential scanning calorimetry (DSC). In this work, the three non-isothermal DSC kinetic approaches including dynamic (Kissinger, Flynn–Wall, and Ozawa); isoconversional (Friedman, Kissinger–Akahira–Sunose (KAS) and Ozawa–Flynn–Wall (OFW)); and Borchardt and Daniels (B/D) methods of data analysis were compared. The kinetic results showed that, under the same conditions, the rate of polymerization for the 7 initiators/initiating systems was in the order of liquid Sn(OnC4H9)2 > Sn(Oct)2/nC4H9OH > Sn(Oct)2 ≅ liquid Sn(OnC6H13)2 > Sn(Oct)2/nC6H13OH ≅ liquid Sn(OnC8H17)2 > Sn(Oct)2/nC8H17OH. The lowest activation energies (Ea = 52, 59, and 56 kJ mol−1 for the Kissinger, Flynn–Wall, and Ozawa dynamic methods; Ea = 53–60, 55–58, and 60–62 kJ mol−1 for the Friedman, KAS, and OFW isoconversional methods; and Ea = 76–84 kJ mol−1 for the B/D) were found in the polymerizations using the novel liquid Sn(OnC4H9)2 as the initiator, thereby showing it to be the most efficient initiator in the ROP of L-lactide.


 Please wait while we load your content...
Please wait while we load your content...