Formation of trisubstituted buta-1,3-dienes and α,β-unsaturated ketones via the reaction of functionalized vinyl phosphates and vinyl phosphordiamidates with organometallic reagents†
Abstract
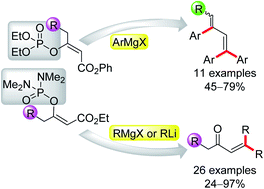
We studied the reactions of vinyl phosphates and vinyl phosphordiamidates containing an ester functional group with organometallic reagents. We found that the functionalized vinyl phosphates were smoothly converted into tri- and tetrasubstituted buta-1,3-dienes via the reaction with aryllithium reagents. Moreover, the vinyl phosphordiamidates were converted into α,β-unsaturated ketones using Grignard reagents. Based on the performed experiments, we proposed a reaction mechanism, which was confirmed by means of the isolation of key intermediates.


 Please wait while we load your content...
Please wait while we load your content...