Oligo-glycerol based non-ionic amphiphilic nanocarriers for lipase mediated controlled drug release†
Abstract
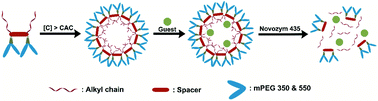
A new class of non-ionic amphiphiles is synthesized using a diaryl derivative of diglycerol as a central core and functionalizing it with long alkyl chains (C-12/C-15) and monomethoxy PEG moiety (Mn: 350/550) by following a chemo-enzymatic approach. The aggregation behavior of the amphiphiles in aqueous medium is studied by using dynamic light scattering (DLS) and fluorescence spectroscopy, whereas the size and morphology of the aggregates are studied by transmission electron microscopy (TEM). A hydrophobic dye, Nile red and a hydrophobic drug, nimodipine, are used to demonstrate the nano-carrier capability of these non-ionic amphiphilic systems and the results are compared with amphiphilic analogues obtained from the triaryl derivatives of triglycerol. The in vitro controlled release of the encapsulated dye is successfully carried out in the presence of immobilized Candida antarctica lipase (Novozym 435). Furthermore, cytotoxicity data is also collected which suggests that the amphiphiles are suitable for biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...