Improving the solubility and bioavailability of anti-hepatitis B drug PEC via PEC–fumaric acid cocrystal†
Abstract
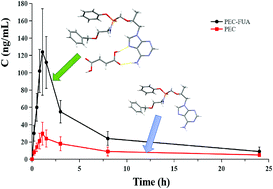
PEC is a new generation of phosphamide ester anti-hepatitis B virus drug. It is a prodrug of tenofovir and can be rapidly metabolized to tenofovir. However, its poor solubility in water (0.219 mg mL−1 at 25 °C) has limited its oral bioavailability. In this study, we aimed to improve the solubility and consequently the oral bioavailability of PEC via a cocrystal. A cocrystal of PEC with fumaric acid (FUA) (PEC–FUA, 1 : 1) was successfully obtained and characterized. The crystal structure of this cocrystal was tested using a single crystal X-ray diffraction method. The intrinsic dissolution rate (IDR) characterization was performed in a pH 6.8 buffer. The solubility of this cocrystal in 0.1 M HCl (pH 1.0) and pH 6.8 phosphate buffers was investigated, and the results showed that the solubility of the cocrystal was 3.8 and 4.0 times that of free PEC, respectively. We also studied the pharmacokinetics of beagle dogs. The mean AUC0–24 h of the cocrystal is about 4.2 times that of free PEC, indicating that the solubility and bioavailability of PEC can indeed be improved by forming the cocrystal. It may become an ideal solid form of an active pharmaceutical ingredient suitable for pharmaceutical preparations, and it can be further studied later.


 Please wait while we load your content...
Please wait while we load your content...