Isolation and characterization of novel acetylcholinesterase inhibitors from Ficus benghalensis L. leaves†
Abstract
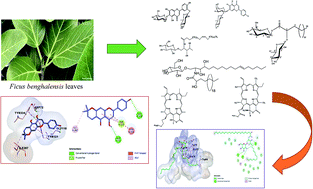
Metabolic profiling of the crude methanolic extract of Ficus benghalensis leaves has revealed the presence of different phenolic and nitrogenous compounds including cerebrosides and tetrapyrrole pigments. A phytochemical study of the ethyl acetate fraction resulted in the identification of three known compounds, namely carpachromene (1), alpha amyrine acetate (2), and mucusoside (3) together with one new fatty acid glycoside, named 2-O-α-L-rhamnopyranosyl-hexacosanoate-β-D-glucopyranosyl ester (4). The compounds were identified using 1D, 2D NMR, and HR-ESIMS techniques as well as via comparison to other literature. Studies on the acetylcholinesterase inhibition potential and antioxidant activity were carried out on the total methanolic leaf extract, ethyl acetate fraction, and the isolated compounds. The results revealed the potent acetylcholinesterase inhibition of mucusoside alongside a new compound. Docking studies were also performed to confirm the possible interaction between the isolated compounds and acetylcholinesterase accompanying Alzheimer's disease progress.


 Please wait while we load your content...
Please wait while we load your content...