Developing non-isocyanate urethane-methacrylate photo-monomers for 3D printing application†
Abstract
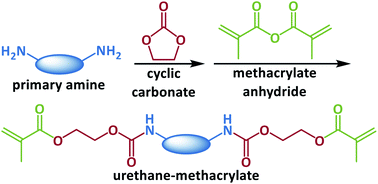
Urethane-methacrylate photo-monomers were prepared via a non-isocyanate route for the 3D printing application. The monomers were synthesized through reacting aliphatic amines, i.e. 1,6-hexanediamine, 1,4-butanediol bis(3-aminopropyl) ether, or n-butylamine, with cyclic carbonates, i.e. ethylene carbonate or propylene carbonate, followed by the methacrylation of the generated hydroxylurethanes. The effects of the chemical structure of monomers on their photo-reactivity and physicomechanical properties of the cured samples were studied. Propylene carbonate generated side methyl groups within the urethane block, which significantly limited the crystallization of the monomers resulting in high photo-reactivity (Rp,max = 6.59 × 10−2 s−1) and conversion (DBCtotal = 85%). The ether bonds of 1,4-butanediol bis(3-aminopropyl) ether decreased the intermolecular hydrogen bonding between urethane blocks, which not only improved the photo-reactivity (Rp,max = 8.18 × 10−2 s−1) and conversion (DBCtotal = 86%) of the monomer but led to a high crosslinking density (νc = 5140 mol m−3) and more flexibility for the cured sample. An ink was developed based on the monomers and successfully 3D printed on a digital light processing machine. In the absence of toxic isocyanates and tin compounds, the non-isocyanate route can be employed to develop urethane-methacrylates with desirable photo-reactivity and physicomechanical properties as good candidates to formulate inks for 3D printing of biomedical materials.


 Please wait while we load your content...
Please wait while we load your content...