One-pot synthesis of formic acid via hydrolysis–oxidation of potato starch in the presence of cesium salts of heteropoly acid catalysts†
Abstract
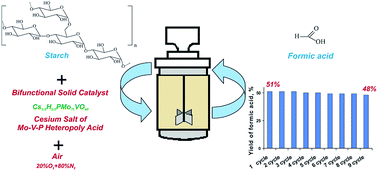
Solid bifunctional catalysts based on cesium salts of V-containing heteropoly acids (CsHPA: Cs3.5H0.5PW11VO40, Cs4.5H0.5SiW11VO40, Cs3.5H0.5PMo11VO40) and Cs2.5H0.5PMo12O40 were used for studying one-pot hydrolysis–oxidation of potato starch to formic acid at 413–443 K and 2 MPa air mixture. It was shown that the optimum process temperature that prevents formic acid from destruction is 423 K. The studies were focused on the influence of the composition of heteropoly anions on the yield and selectivity of formic acid. Using W–V–P(Si) CsHPA results in the product overoxidation compared to Mo–V-containing CsHPA. The activity of Cs–PMo was significantly lower compared to Cs–PMoV. This may indicate that vanadium plays an important role in the oxidation process. The most promising catalyst was Cs3.5H0.5PMo11VO40 which provided the maximum yield of formic acid equal to 51%. Cs3.5H0.5PMo11VO40 was tested during nine cycles of starch hydrolysis–oxidation to demonstrate its high stability and efficiency.


 Please wait while we load your content...
Please wait while we load your content...