A hierarchical Ca/TiO2/NH2-MIL-125 nanocomposite photocatalyst for solar visible light induced photodegradation of organic dye pollutants in water†
Abstract
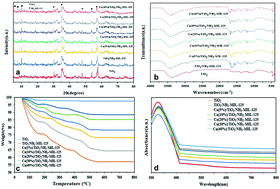
In this study, for the first time, the Ca/TiO2/NH2-MIL-125 nanocomposite photocatalyst was synthesized for the purpose of photodegradation of Methyl Orange (MO) and Rhodamine B (RhB) dyes under visible light irradiation. The structural and chemical properties of the nanocomposite photocatalyst were characterized through FTIR, XRD, TGA, PL, XPS, ICP-OES and UV-DRS. For the photodegradation efficiency analysis, the effect of pH (3, 5, 7, 9, and 11), photocatalyst dosage (0.1, 0.2, 0.4, 0.6, and 0.8 g L−1), dye concentration (1–40 mg L−1), and contact time (10–120 min) was precisely evaluated. The largest photodegradation efficiency for RhB and MO dye models was 82.87% and 86.22%, respectively, that was obtained under optimal conditions in terms of pH and photocatalyst dosage and for Ca(30%)/TiO2/NH2-MIL-125. The photodegradation process of the dyes complied well with the first-order kinetic model. Moreover, the nanocomposite photocatalyst showed consistent photodegradation efficiency and after 6 successive cycles with fresh dye solutions, it could still perform comparably well. Taken together, Ca/TiO2/NH2-MIL-125 photocatalyst is able to show a high photodegradation efficiency for dye pollutants and optimum stability and reusability.


 Please wait while we load your content...
Please wait while we load your content...