The impact of alicyclic substituents on the extraction ability of new family of 1,10-phenanthroline-2,9-diamides†
Abstract
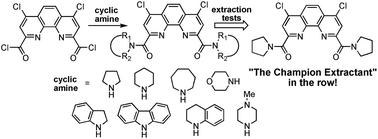
Development of efficient extractants for the separation of actinides and lanthanides in the technologies of nuclear fuel cycle is one of the most urgent and complex tasks in modern nuclear energetics. New family of 4,7-dichloro-1,10-phenanthroline-2,9-dicarboxylic acid diamides based on cyclic amines was synthesized and shown to exhibit high selectivity in the La/Am pair separation (SF (Am/La ≈ 10)) and in the Am/Eu pair separation (SF (Am/Eu ≈ 12)). It was shown that pyrrolidine derived diamide is more efficient extractant for americium, curium and lanthanides from highly acidic HNO3 solution than its non-cyclic N,N,N′,N′-tetraalkyl analogues. The structures of synthesized compounds were studied in details by IR, NMR spectroscopy, and single crystal X-ray diffraction. According to spectroscopy data, incorporation of aromatic rings to the amide fragment of ligand leads to complex dynamic behavior in solutions what is believed to strongly affect the extraction ability of synthesized ligands.
- This article is part of the themed collection: Shining a Light on the f-Block


 Please wait while we load your content...
Please wait while we load your content...