Fluorescence enhancement of quinolines by protonation†
Abstract
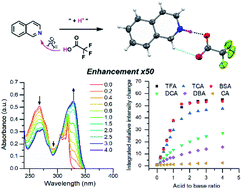
A study of the fluorescence enhancement of isoquinoline, acridine (benzo[b]quinoline) and benzo[h]quinoline is reported with six organic acids of different pKa values. Protonation was found to be an effective tool in the fluorescence enhancement of quinolines. A significant increase in the fluorescence intensity is observed only when strong acids are used, resulting in an over 50-fold increase in fluorescence with trifluoroacetic or benzenesulfonic acid and isoquinoline in a 1.5 : 1 ratio. The benzenesulfonic acid was found to be the most effective in the protonation of the bases despite its higher pKa value compared to trifluoro- and trichloroacetic acid. The X-ray crystal structures of 14 salts reveal the charge-assisted hydrogen bond O⋯N distances to vary very little, from 2.560(2)–2.714(3) Å, with the exception of the isoquinolinium benzenesulfonate where the O⋯N distance of 2.862(7) Å is caused by additional intermolecular interactions in the solid-state.


 Please wait while we load your content...
Please wait while we load your content...