Revision of the structure of isochaetoglobosin Db based on NMR analysis and biosynthetic consideration†
Abstract
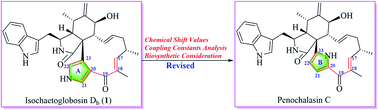
Isochaetoglobosin Db is a new chaetoglobosin possessing a unique 3,4-substituted pyrrole ring isolated and named by Qiu et al., and it is different from any one of the 14 sub-types in the macrocyclic ring of chaetoglobosins classified in our previous work. Its chemical shift values, coupling constants and biosynthetic consideration implied that the proposed structure of isochaetoglobosin Db was incorrect. In this report, based on detailed NMR data analysis together with biosynthetic consideration, the structure of isochaetoglobosin Db is suggested to be revised to that of penochalasin C. The NMR spectra of penochalasin C measured in the same solvent (DMSO-d6) as that of isochaetoglobosin Db supported the above conclusion. The results imply that reasonable biosynthetic consideration could complement spectroscopic structural determination, and also support that the 1H-NMR rule of chaetoglobosin summarized in our previous work can provide help for dereplication and rectification.


 Please wait while we load your content...
Please wait while we load your content...