Transition metal-free domino acyl substitution/Michael addition of alkenyl Grignard reagents to lactam esters: synthesis of lactam-bearing homoallylic ketones†
Abstract
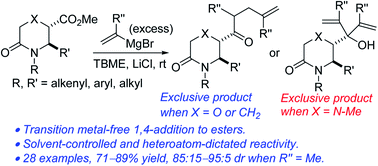
A solvent-controlled protocol for the direct and transition metal-free addition of alkenyl Grignard reagents to vicinally functionalized sp3-rich morpholinones has been developed, leading to the chemo and regioselective synthesis of lactam-bearing homoallylic ketones. The addition of lithium chloride proved to be essential. In cases where a new stereocenter is generated, the doubly branched homoallylic ketones are obtained in unexpectedly high diastereoselectivities. Efforts to extend the methodology to other heterosubstituted lactams revealed some important reactivity and selectivity differences.


 Please wait while we load your content...
Please wait while we load your content...