Residue-based propensity of aggregation in the Tau amyloidogenic hexapeptides AcPHF6* and AcPHF6†
Abstract
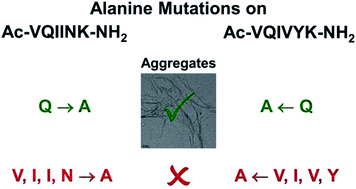
In Alzheimer's disease and related tauopathies, the aggregation of microtubule-associated protein, Tau, into fibrils occurs via the interaction of two hexapeptide motifs PHF* 275VQIINK280 and PHF 306VQIVYK311 as β-sheets. To understand the role of the constituent amino acids of PHF and PHF* in the aggregation, a set of 12 alanine mutant peptides was synthesized by replacing each amino acid in PHF and PHF* with alanine and they were characterized by nuclear magnetic resonance (NMR) spectroscopy, circular dichroism (CD), transmission electron microscopy (TEM) and ThS/ANS fluorescence assay. Our studies show that while the aggregation was suppressed in most of the alanine mutant peptides, replacement of glutamine by alanine in both PHF and PHF* enhanced the fibrillization.


 Please wait while we load your content...
Please wait while we load your content...