Synthetic and biochemical studies on the effect of persulfidation on disulfide dimer models of amyloid β42 at position 35 in Alzheimer's etiology†
Abstract
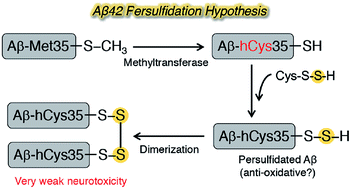
Protein persulfidation plays a role in redox signaling as an anti-oxidant. Dimers of amyloid β42 (Aβ42), which induces oxidative stress-associated neurotoxicity as a causative agent of Alzheimer's disease (AD), are minimum units of oligomers in AD pathology. Met35 can be susceptible to persulfidation through its substitution to homoCys residue under the condition of oxidative stress. In order to verify whether persulfidation has an effect in AD, herein we report a chemical approach by synthesizing disulfide dimers of Aβ42 and their evaluation of biochemical properties. A homoCys-disulfide dimer model at position 35 of Aβ42 formed a partial β-sheet structure, but its neurotoxicity was much weaker than that of the corresponding monomer. In contrast, the congener with an alkyl linker generated β-sheet-rich 8–16-mer oligomers with potent neurotoxicity. The length of protofibrils generated from the homoCys-disulfide dimer model was shorter than that of its congener with an alkyl linker. Therefore, the current data do not support the involvement of Aβ42 persulfidation in Alzheimer's disease.


 Please wait while we load your content...
Please wait while we load your content...