Oscillating syngas production on NiO/YSZ catalyst from methane oxidation†
Abstract
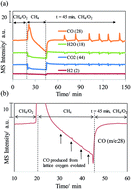
Synthesis gas was produced by methane oxidation on a NiO/YSZ cermet by interrupting the oxygen flow. Stopping the oxygen flow provoked the diffusion of lattice oxygen in the cermet, which in turn replenished the Ni–O bond that was consumed by methane. Resuming the oxygen flow brought about the activation of oxygen on the extrinsic vacancy site of YSZ. The activation, followed by the diffusion of oxygen and a Ni/NiO redox cycle, led to oscillatory syngas production. The infrared and mass spectroscopy results provide the reaction mechanism that governs the oxidation of methane on the NiO/YSZ cermet. This study presents a technique that can be applied to the catalysis of other metal/anion or cation conductor systems.


 Please wait while we load your content...
Please wait while we load your content...