N-Amino peptide scanning reveals inhibitors of Aβ42 aggregation†
Abstract
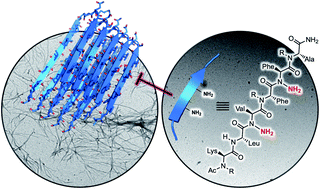
The aggregation of amyloids into toxic oligomers is believed to be a key pathogenic event in the onset of Alzheimer's disease. Peptidomimetic modulators capable of destabilizing the propagation of an extended network of β-sheet fibrils represent a potential intervention strategy. Modifications to amyloid-beta (Aβ) peptides derived from the core domain have afforded inhibitors capable of both antagonizing aggregation and reducing amyloid toxicity. Previous work from our laboratory has shown that peptide backbone amination stabilizes β-sheet-like conformations and precludes β-strand aggregation. Here, we report the synthesis of N-aminated hexapeptides capable of inhibiting the fibrillization of full-length Aβ42. A key feature of our design is N-amino substituents at alternating backbone amides within the aggregation-prone Aβ16–21 sequence. This strategy allows for maintenance of an intact hydrogen-bonding backbone edge as well as side chain moieties important for favorable hydrophobic interactions. An N-amino scan of Aβ16–21 resulted in the identification of peptidomimetics that block Aβ42 fibrilization in several biophysical assays.


 Please wait while we load your content...
Please wait while we load your content...