Polymorphism of Au11(PR3)7Cl3 clusters: understanding C–H⋯π interaction and C–H⋯Cl–C van der Waals interaction on cluster assembly by surface modification†
Abstract
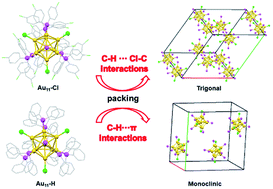
The C–H⋯π interaction and the C–H⋯Cl–C van der Waals interaction play a crucial role in the crystallization of nanoclusters. In this paper, we present an example of a crystal system transformation of Au11(PR3)7Cl3 from monoclinic (M) to trigonal (T) by surface modification. Atomically-resolved gold nanoclusters containing tris(4-chlorophenyl)phosphine and chloride ligands were synthesized and determined to be Au11(p-ClPPh3)7Cl3 (p-ClPPh3 = tris(4-chlorophenyl)phosphine) by X-ray crystallography. Crystal data demonstrated that the C–H⋯Cl–C interaction is dominant in a trigonal crystal system of Au11(p-ClPPh3)7Cl3 with a R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group. However, the C–H⋯π interaction is the major driving force to form a monoclinic crystal system of Au11(PPh3)7Cl3 (PPh3 = triphenylphosphine) with a P2(1)/n space group. Moreover, UV-vis absorption spectra and X-ray photoelectron spectra reveal that the electronic structure of the Au11(p-ClPPh3)7Cl3 nanocluster is greatly influenced by p-ClPPh3. This work provides critical implications for the crystallization of metal nanoclusters, as well as a better understanding of the non-covalent interaction on the nanocluster assembly and the crystal engineering by surface modification.
space group. However, the C–H⋯π interaction is the major driving force to form a monoclinic crystal system of Au11(PPh3)7Cl3 (PPh3 = triphenylphosphine) with a P2(1)/n space group. Moreover, UV-vis absorption spectra and X-ray photoelectron spectra reveal that the electronic structure of the Au11(p-ClPPh3)7Cl3 nanocluster is greatly influenced by p-ClPPh3. This work provides critical implications for the crystallization of metal nanoclusters, as well as a better understanding of the non-covalent interaction on the nanocluster assembly and the crystal engineering by surface modification.


 Please wait while we load your content...
Please wait while we load your content...