Histidine-conjugated DNA as a biomolecular depot for metal ions†
Abstract
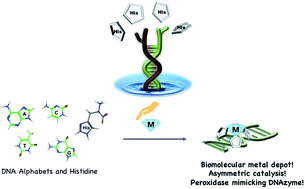
Histidine is a versatile amino acid residue that plays a critical role in the active sites of many metalloenzymes. DNA is an attractive biomolecular scaffold owing to its chemical and thermal stability and easy accessibility. Herein, we report histidine-conjugated DNA oligonucleotides, which were synthesized by combining DNA alphabets and natural metal-binding amino acids, as novel biohybrid materials and demonstrate their use as molecular depots for various metal ions. Moreover, histidine-conjugated DNA oligonucleotides could be successfully used in asymmetric catalysis (up to 90% conversion and 95% ee) as DNA metalloenzymes and in 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) oxidation reactions as horseradish-peroxidase (HRP)-mimicking DNAzymes with suitable metal cofactors. Nature-inspired histidine-DNA hybrids will become an attractive strategy to construct fine-tuned coordination environments as an alternative to bioremediation and the development of multimetal enzymes.


 Please wait while we load your content...
Please wait while we load your content...