Degradation of p-nitrophenol by nano-pyrite catalyzed Fenton reaction with enhanced peroxide utilization†
Abstract
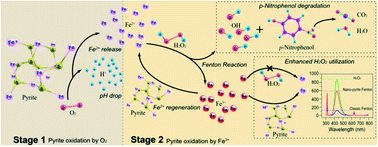
Pyrite (FeS2) catalyzed conversion of H2O2 into oxidants is increasingly recognized as a promising Fenton-like process for treating recalcitrant contaminants. However, the underlying mechanism remains unclear, especially for nano-pyrite. The present study explored the potential of a nano-pyrite Fenton system for p-nitrophenol oxidation using high energy ball milled nano-pyrite. The enhancement in ˙OH production, with 3 times faster p-nitrophenol degradation than the conventional Fenton system, is ascribed to the reduction of pyrite size to the nanoscale, which alters the Fe2+ regeneration pathway, favoring faster and very efficient production of ˙OH during H2O2 decomposition. The amount of H2O2 required was reduced due to the increased conversion efficiency of H2O2 to ˙OH from 13.90% (conventional Fenton) to 67.55%, in which surface S22− species served as an electron source. An interpretation of the degradation intermediates and mineralization pathway of p-nitrophenol was then made using gas chromatography-mass spectrometry. This study bridges the knowledge gap between p-nitrophenol removal and the nano-pyrite catalyzed oxidant generation process.


 Please wait while we load your content...
Please wait while we load your content...