Treasures old and new: what we can learn regarding the macrocyclic problem from past and present efforts in natural product total synthesis
Abstract
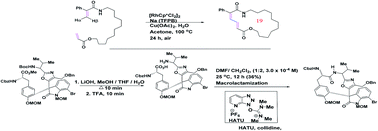
In this review the strategies leading to successful macrocyclization, in the context of total synthesis are discussed. These synthetic endeavors will be discussed paying particular attention to the methods employed, and including the type of reactive intermediates that could play a key role in key cyclization steps. In many cases “simple” macrocyclization methods were found to be inadequate, and alternative creative approaches were required. For example, we describe Boger's imaginative development of the intramolecular version of the Larock annulation which yielded the chloropeptin 1 DEF macrocycle. Peptide coupling approaches were unsuccessful. In another example, a key macrocyclic domain within diazonamide was beautifully installed (Nicolaou, et al.) by single electron oxidation/reduction (Witkop reaction), thereby establishing a crucial biaryl functionality. In contrast, oxidative methodologies failed to deliver the distorted biaryl found in haouamine, and Baran, et al. subsequently exploited a spectacular pyrone N-butyne intramolecular Diels–Alder reaction to install this biaryl moiety. Other unexpected and mechanistically intriguing observations will be described throughout the review.
- This article is part of the themed collection: 2020 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...