Functionalization of C–H bonds in acetophenone oximes with arylacetic acids and elemental sulfur†
Abstract
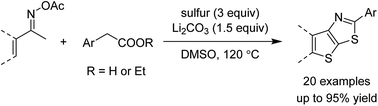
Fused thieno[3,2-d]thiazoles were synthesized via a coupling of acetophenone ketoximes, arylacetic acids, and elemental sulfur in the presence of Li2CO3 base. Functionalities including chloro, bromo, fluoro, trifluoromethyl, and pyridyl groups were compatible with reaction conditions. High yields and excellent regioselectivities were obtained even if meta-substituted ketoxime acetates were used. Ethyl esters of heteroarylacetic acids were competent substrates, which is very rare in the literature. Our method would offer a convenient protocol to afford polyheterocyclic structures from simple substrates.


 Please wait while we load your content...
Please wait while we load your content...