Understanding the correlation between structure and dynamics of clocortolone pivalate by solid state NMR measurement
Abstract
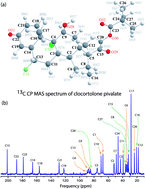
Structural characteristics of clocortolone pivalate are unique in the topical corticosteroid field having high penetration power through the stratum corneum of skin as well as low corticosteroid-related adverse effects. The molecule was thoroughly studied by 13C 2DPASS CP MAS NMR and spin–lattice relaxation time measurements. Molecular correlation time at different carbon nuclei positions was calculated by assuming that the chemical shift anisotropy interaction and heteronuclear dipole–dipole interaction play vital roles in the 13C spin–lattice relaxation mechanism. The CSA parameters are substantially varied at different carbon nuclei sites. This suggests that the electronic distribution surrounding the carbon nuclei varies widely when the same carbon atom is placed in different chemical surroundings of the molecule. The spinning CSA sideband pattern for C11, C17, C26 nuclei is axially symmetric. The asymmetry parameter is very small (≤0.3) for C2, C5, C10, C22, C23, C24 nuclei, and it is reasonably high (≥0.9) for C3, C4, C6, C18, C19, C21 nuclei. The anisotropy parameter is very high for double bonded C14, C15, C18, C19, C21, C22, and C23 nuclei. Spin–lattice relaxation time and molecular correlation time are also varied substantially for carbon nuclei situated at various positions of the molecule. The spin–lattice relaxation time is slow for carbon nuclei residing at the carbon ring, and it is very fast for C12, C17, C16, C26 carbon nuclei situated at the side portion of the molecule. Molecular correlation time is of the order of 10−4 s for those carbon nuclei attached with neighbouring carbon or oxygen atoms by double bonds like C14, C15, C18, C19, C21, C22, and C23. It implies that the molecular correlation time is very high for those carbon nuclei associated with high values of the chemical shift anisotropy parameter. In contrast, the molecular correlation time is of the order of 10−8 s for C12, C16, and C17 carbon nuclei. From these studies, it is clear that the various portions of the molecule exhibit different degrees of motion and the dynamics is related with the structural characteristics of the molecule. These investigations on important drug clocortolone pivalate by solid state NMR will help researchers to understand the structure and dynamics of the molecule, which will give a direction to develop advance corticosteroids.


 Please wait while we load your content...
Please wait while we load your content...