Diels–Alder reactions between cyclopentadiene analogs and benzoquinone in water and their application in the synthesis of polycyclic cage compounds†
Abstract
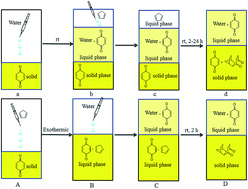
Diels–Alder reactions between cyclopentadiene analogs and p-benzoquinone were explored in water and yielded 83–97% product, higher than the results reported in water with a catalyst or cetrimonium bromide (CTAB) micelles. The novel adduct 10 was synthesized and further used to synthesize the bi-cage hydrocarbon 4,4′-spirobi[pentacyclo[5.4.0.02,6.03,10.05,9]undecane], which has a high density (1.2663 g cm−3) and a high volumetric heat of combustion (53.353 MJ L−1). Four novel bi-cage hydrocarbon compounds were synthesized in water using this method starting from 2,2′-bi(p-benzoquinone) and cyclopentadiene analogs.


 Please wait while we load your content...
Please wait while we load your content...