Effects of metal–organic complex Ni(Salen) on thermal decomposition of 1,1-diamino-2,2-dinitroethylene (FOX-7)†
Abstract
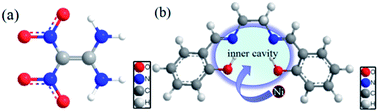
The development of novel combustion catalysts is critical for improving combustion performance of high-energy solid propellants. In this work, a nickel-based Schiff base complex, N,N′-bis(salicylidene)ethylenediamino nickel [Ni(Salen)], as a candidate for the combustion catalyst of solid propellants, was synthesized with high purity. The effects of Ni(Salen) on the thermal decomposition reaction of FOX-7, which is closely related to its combustion properties in propellants, were systematically investigated and the corresponding mechanisms were also evaluated through comparison and analysis. Under the action of Ni(Salen), peak temperatures of the two decomposition processes of FOX-7 were reduced from 235.7 and 296.3 °C to 233.7 and 268.7 °C, respectively. The investigations into the mechanisms revealed the interactions between the Schiff base skeleton in Ni(Salen) and FOX-7, which promotes the decomposition of the remaining FOX-7. In addition, it was found that NiO, which was a decomposition product of Ni(Salen), also played an important role in promoting the thermal decomposition of FOX-7.


 Please wait while we load your content...
Please wait while we load your content...