R-VAPOL-phosphoric acid based 1H and 13C-NMR for sensing of chiral amines and acids†
Abstract
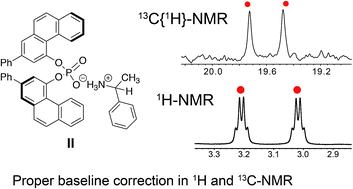
Enantiomers have significant importance in pharmaceuticals, biology and modern chemistry and therefore distinguishing and quantifying the enantiomeric forms is of utmost importance. Herein, we propose diphenyl-3,3′-biphenanthryl-4,4′-diyl phosphate (R-VAPOL-PA) as a promising chiral solvating agent to discriminate amines and acids of poly-functional groups such as chiral amines, amino alcohols and hydroxy acids. The methodological approach involves using the nature of hydrogen bonds and ion pairs as a mode of weak interactions to form diastereomers where the probe is associated with enantiomers. The resulting diastereomer difference in the NMR spectrum enables the chiral discrimination with a complete baseline peak separation and an accurate enantiomeric excess (ee) analysis. We also carried out density functional theory (DFT) calculations to understand the complex formation to explain enantiodiscrimination by analysing the formation and stability of different chiral complexes. The binding energy differences between enantiomeric forms revealed by DFT calculations are qualitatively in agreement with the diastereomer difference in the NMR spectrum and unequivocally establishes the suggested experimental protocol of R-VAPOL-PA-based enantiomeric discrimination.


 Please wait while we load your content...
Please wait while we load your content...