The influences of electronic effect and isomerization of salalen titanium(iv) complexes on ethylene polymerization in the presence of methylaluminoxane†
Abstract
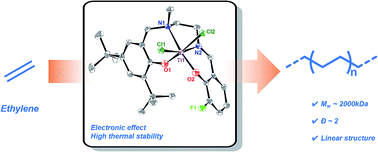
Herein, two salalen titanium(IV) complexes were synthesized and characterized. These complexes coexisted as two isomers in certain conditions and underwent isomerization, as evidenced by 1H NMR spectroscopy. Furthermore, the molar ratio of the two isomers ranged from 100 : 15 at 30 °C to 100 : 34 at 120 °C, driven by thermal energy, based on variable temperature 1H NMR characterization. Both complexes were employed as catalysts for ethylene polymerization in the presence of methylaluminoxane (MAO). The influence of the electronic effects of different substituent groups at the ortho position of the phenolate on ethylene polymerization behaviors, molecular weight and molecular weight distributions of the resulting polyethylene was investigated. The fluorinated salalen titanium(IV) complex revealed relatively high catalytic activity and thermal stability owing to the electron-withdrawing inductive effect. Moreover, disentangled linear polyethylene with ultrahigh molecular weight (Mw up to 3000 kDa) and narrow molecular weight distribution (Mw/Mn ∼ 2) was obtained in the polymerization temperature range of 30 °C to 50 °C.


 Please wait while we load your content...
Please wait while we load your content...