Electrocatalytic water oxidation by a Ni(ii) salophen-type complex†
Abstract
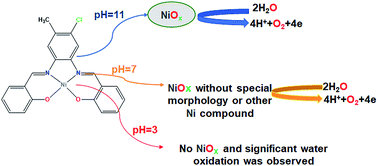
A new mononuclear Ni(II) complex, NiL (1), was synthesized from the reaction of Ni(OAc)2·4H2O and salophen-type N2O2-donor ligand, H2L (where H2L = 2,2′-((1E,1′E)-((4-chloro-5-methyl-1,2-phenylene)bis(azanylylidene))bis(methanylylidene))diphenol), in ethanol. The obtained complex was characterized by elemental analysis, spectroscopic techniques and single crystal X-ray analysis. The complex was studied as a water oxidizing catalyst and its electrocatalytic activity in the water oxidation reaction was tested in 0.5 M of borate buffer at pH = 3, 7 and 11 in a typical three-electrode setup with a carbon paste electrode modified by complex 1 as a working electrode. The linear sweep voltammetry (LSV) curves indicated that complex 1 has a much superior activity and only needs 21 mV vs. Ag/AgCl overvoltage to reach a geometrical catalytic current density of 2.0 mA cm−2 at pH = 11. The onset potential decreased from 1.15 V to 0.67 V vs. Ag/AgCl with an increase of pH from 3 to 13 under a constant current density of 1.0 mA cm−2. Then, to determine the true catalyst for the water oxidation reaction in the presence of complex 1 at pH = 3, 7 and 11, cyclic voltammetry was also performed. The continuous CVs for complex 1 at neutral and alkaline solutions showed significant progress for the water oxidation reaction. In addition, the amperometry tests exhibited excellent stability and high constant current density for water oxidation by CPE-complex 1 under electrochemical conditions at pH = 11 and 7. Although X-ray powder diffraction analysis did not show a pure and crystalline structure for NiOx, the scanning electron microscopy images showed that nickel oxide at pH = 11 and nickel oxide or other Ni-based compounds at pH = 7 are true water oxidizing catalysts on the surface of a CPE electrode. Moreover at pH = 3, no clear water oxidation or NiOx formation was observed.


 Please wait while we load your content...
Please wait while we load your content...