Effect of extremely high CO2 pressure on the formation of the corrosion film on 13Cr stainless steel
Abstract
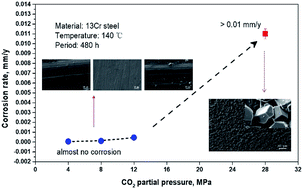
The corrosion behaviors of 13Cr martensitic steel under different CO2 partial pressures (4–28 MPa) were investigated by weight loss tests and surface characterizations. The results show that the corrosion rate of 13Cr steel shows a sharp increase under higher CO2 pressure (28 MPa), which reached approximately 20–180 times as large as those under lower CO2 pressures (4–12 MPa). Under the lower CO2 pressures, a single-layered Cr(OH)3 passive film forms and completely covers the steel surface. However, when the CO2 pressure reaches 28 MPa, a very different corrosion film which contains an inner Cr(OH)3 passive layer and an outer FeCO3 layer forms, and the inner passive layer shows local damage. This phenomenon can be explained by the lower pH (∼2.75) and the higher H2CO3 concentration in the solution under the higher CO2 pressure.


 Please wait while we load your content...
Please wait while we load your content...