Exploring the potentials of Ti3N2 and Ti3N2X2 (X = O, F, OH) monolayers as anodes for Li or non-Li ion batteries from first-principles calculations†
Abstract
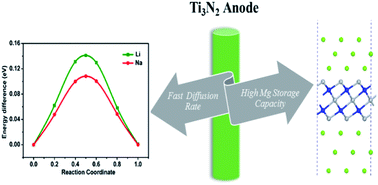
The electronic properties and different metal ion (Li, Na, Mg) storage capabilities of the two-dimensional (2D) Ti3N2 monolayer and its Ti3N2X2 derivatives (X = O, F, and OH) as anode materials in rechargeable batteries have been systematically investigated by density functional theory (DFT) computations. Results show that the bare Ti3N2 and terminated monolayers in their most stable configurations are all metallic before and after metal ion adsorption. The relatively low diffusion barriers on the bare Ti3N2 monolayer were also confirmed, which implies faster charge and discharge rates. With respect to storage capacity, a high theoretical capacity of 1874 mA h g−1 can be provided by the Ti3N2 monolayer for Mg due to its multilayer adsorption and two-electron reaction. The existence of functional groups is proven to be unfavorable to metal ion migration and will decrease the corresponding storage capacities, which should be avoided in experiments as much as possible. These excellent performances suggest that the bare Ti3N2 is a promising anode material for Li-ion or non-Li-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...