A bio-inspired synthesis of hybrid flavonoids from 2-hydroxychalcone driven by visible light†
Abstract
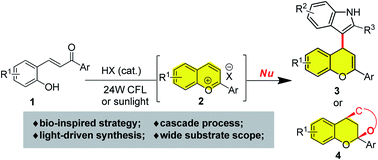
A bio-inspired synthesis of hybrid flavonoids from 2-hydroxylchalcone is described. Under the irradiation of 24 W CFL, 2-hydroxychalcone reacts with various nucleophiles to deliver structurally diverse hybrid flavonoids in good to excellent yields in the presence of a catalytic Brønsted acid. Moreover, moderate enantioselectivities could be obtained using a catalytic chiral phosphoric acid via counter anion directed addition. Based on mechanistic studies, the reaction is proposed to proceed via tandem double-bond isomerization/dehydrated cyclization of 2-hydroxychalcone to form a transient flavylium cation, which is in situ captured by nucleophiles to afford hybrid flavonoids.


 Please wait while we load your content...
Please wait while we load your content...