Graphene chemiresistors modified with functionalized triphenylene for highly sensitive and selective detection of dimethyl methylphosphonate†
Abstract
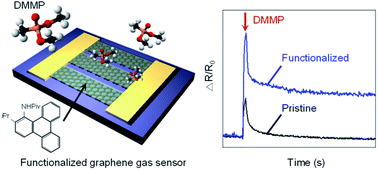
Graphene has attracted significant attention from researchers in recent years as a gas sensing material, because of its atom-thick 2-D structure, extremely high surface-to-volume ratio, and high carrier mobility. However, chemiresistive gas sensors based on graphene have a drawback of low sensitivity to organophosphates, including dimethyl methylphosphonate (DMMP), a simulant of the nerve agent sarin. In this study, we report the detection of 1.3 ppm DMMP, the highest sensitivity reported to date, using graphene chemiresistors, by non-covalently functionalizing graphene with N-substituted triphenylene. The functionalized graphene sensor exhibits a two orders of magnitude higher response to DMMP than to other compounds. This high sensitivity and selectivity are attributed to the strong hydrogen bonding between DMMP and N-substituted triphenylene, as well as the hole-doping effect caused by triphenylene, which increases the binding affinity to the electron-donating DMMP. The proposed approach for simple functionalization of graphene with substituted triphenylene can potentially be employed in tuning the properties of other conjugated nanomaterials, such as carbon nanotubes and graphene nanoribbons, to detect various target analytes.


 Please wait while we load your content...
Please wait while we load your content...