Enzymatic synthesis of biobased aliphatic–aromatic oligoesters using 5,5′-bis(hydroxymethyl)furoin as a building block†
Abstract
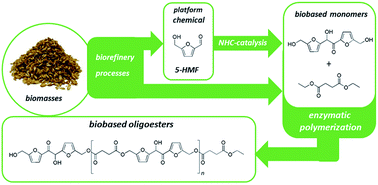
5,5′-Dihydroxymethyl furoin (DHMF) is a novel biobased difuranic polyol scaffold, achievable from the benzoin condensation of 5-hydroxymethylfurfural (HMF), which has recently been employed as a monomer for the preparation of cross-linked polyesters and polyurethane. Its upgrading by means of enzymatic reactions has not yet been reported. Here we demonstrated that Candida antarctica lipase B (CALB) is a suitable biocatalyst for the selective esterification of the primary hydroxyl groups of DHMF. Exploiting this enzymatic activity, DHMF has been reacted with the diethyl esters of succinic and sebacic acids obtaining fully biobased linear oligoesters with number-average molecular weight around 1000 g mol−1 and free hydroxyl groups on the polymer backbone. The structures of the DHMF-diacid ethyl ester dimers and of the oligomers were elucidated by NMR and MS analyses.


 Please wait while we load your content...
Please wait while we load your content...