Electrochemical fabrication of FeSx films with high catalytic activity for oxygen evolution
Abstract
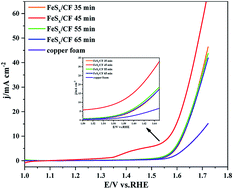
Electrochemical decomposition of water to produce oxygen (O2) and hydrogen (H2) through an anodic oxygen evolution reaction (OER) and a cathodic hydrogen evolution reaction (HER) is a promising green method for sustainable energy supply. Here, we demonstrate that cauliflower-like S-doped iron microsphere films are materials that can efficiently decompose water as an electrocatalyst for the oxygen evolution reaction. FeSx films are prepared by a simple one-step electrodeposition method and directly grow on copper foam from a deep eutectic solvent, ethaline (mixture of choline chloride and ethylene glycol), as a durable and highly efficient catalyst for the OER in 1.0 M KOH. The prepared FeSx/CF, as an oxygen-evolving anode, shows remarkable catalytic performance toward the OER with a moderate Tafel slope of 105 mV dec−1, and require an overpotential of only 340 mV to drive a geometrical catalytic current density of 10 mA cm−2. In addition, this catalyst also demonstrates strong long-term electrochemical durability. This study provides a simple synthesis route for practical applications of limited transition metal nano catalysts.


 Please wait while we load your content...
Please wait while we load your content...