Kinetics and pathways of diclofenac degradation by heat-activated persulfate
Abstract
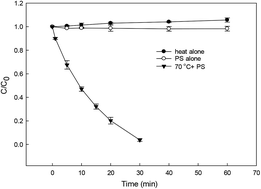
In this study, the degradation of diclofenac (DCF) by heat-activated persulfate (HAP) was investigated. It was found that DCF could be degraded efficiently by HAP. The degradation of DCF followed the pseudo-first-order kinetic model, and the highest observed degradation rate constant (kobs) was obtained at pH 3. The sulfate radical was mainly responsible for DCF removal at pH < 7, whereas it was the hydroxyl radical at high pH. The elimination of DCF was enhanced with the increase in temperature or initial dosage of persulfate. Presence of Cu2+ and CO32− could improve DCF degradation, while an inhibition effect was observed in the presence of natural organic matter. According to the identified nine transformation products, the potential DCF degradation mechanism was proposed revealing five different reaction pathways, including hydroxylation, decarboxylation, formylation, dehydrogenation and C–N bond cleavage. This study indicates that HAP can effectively oxidize and degrade DCF, especially under acidic conditions.


 Please wait while we load your content...
Please wait while we load your content...