Preparation and characterization of a nanolignin phenol formaldehyde resin by replacing phenol partially with lignin nanoparticles
Abstract
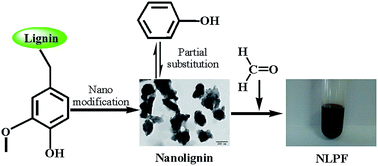
A new strategy for the preparation of a lignin phenol formaldehyde (LPF) resin has been developed. Nanolignin with high specific surface area and porous structure with an average particle size of about 300 nm was prepared, used as the raw material to substitute phenol partially, and combined with formaldehyde to produce a wood adhesive. The results show that the artificial board prepared with a nanolignin phenol formaldehyde (NLPF) resin with nanolignin substitution degree of 40% wt for phenol could give a dry bond strength of 1.30 ± 0.08 MPa, which is 1.85 times that of the Chinese national grade 1 plywood standard (0.7 MPa) and whose formaldehyde emission of 0.40 mg L−1 meets the standard of GB/T 14732-2006 (E0, 0.5 mg L−1). TG and DSC analyses show that the replacement of phenol by nanolignin could improve the thermal stability and decrease the curing temperature of the prepared lignin-based resin, with the residual ratio of 40% NLPF being 45% wt at 800 °C and the curing exothermic peak being 145.4 °C, which are much better than that of the 40% LPF resin with the residual ratio being 40% wt and the exothermic peak being 186 °C, respectively. The present study provides a new thought for preparation of LPF resins.


 Please wait while we load your content...
Please wait while we load your content...