Phosphonic acid mediated practical dehalogenation and benzylation with benzyl halides†
Abstract
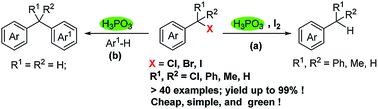
For the first time, by using H3PO3/I2 system, various benzyl chlorides, bromides and iodides were dehalogenated successfully. In the presence of H3PO3, benzyl halides underwent electrophilic substitution reactions with electron-rich arenes, leading to a broad range of diarylmethanes in good yields. These transformations feature green, cheap reducing reagents and metal-free conditions. A possible mechanism was proposed.


 Please wait while we load your content...
Please wait while we load your content...