Synthesis of C2-tetrasubstituted indolin-3-ones via Cu-catalyzed oxidative dimerization of 2-aryl indoles and cross-addition with indoles†
Abstract
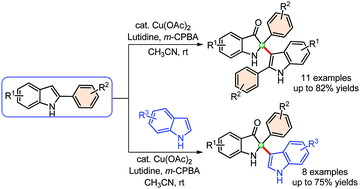
An efficient protocol for the synthesis of 2,2-disubstituted indolin-3-ones under mild conditions has been developed. This reaction involves the copper-catalyzed in situ oxidative de-aromatization of 2-arylindoles to indol-3-one, followed by self-dimerization as well as cross-addition with indoles under mild conditions. The result generates a wide variety of C2-tetrasubstituted indolin-3-ones with good to high yields (62–82%).
- This article is part of the themed collection: Elegant Synthetic Routes to Indole Derivatives


 Please wait while we load your content...
Please wait while we load your content...