Utilization of catecholic functionality in natural safrole and eugenol to synthesize mussel-inspired polymers†
Abstract
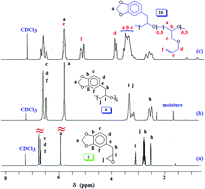
Naturally occurring safrole I upon epoxidation gave safrole oxide II, which underwent ring opening polymerization using a Lewis acid initiator/catalyst comprising of triphenylmethylphosphonium bromide/triisobutylaluminum to afford new polyether III in excellent yields. Epoxy monomer II and allyl glycidyl ether IV in various proportions have been randomly copolymerized to obtain copolymer V. A mechanism has been proposed for the polymerization reaction involving chain transfer to the monomers. A strategy has been developed for the deprotection of the methylene acetal of V using Pb(OAc)4 whereby one of the methylene protons is replaced with a labile OAc group to give VI. The pendant allyl groups in VI have been elaborated via a thiol–ene reaction using cysteamine hydrochloride and thioglycolic acid to obtain cationic VII and anionic VIII polymers, both containing a mussel-inspired Dopa-based catechol moiety. During aqueous work up, the protecting group containing OAc was deprotected under mild conditions. Cationic VII and anionic VIII were also obtained via an alternate route using epoxide IX derived from 3,4-bis[tert-butyldimethylsilyloxy]allylbenzene. Monomer IX was homo- as well as copolymerized with IV using Lewis acid initiator/catalyst system to obtain homopolymer X and copolymer X1. Copolymer XI was then elaborated using a thiol–ene reaction followed by F− catalysed silyl deprotection to obtain mussel inspired polymers VII and VIII, which by virtue of having charges of opposite algebraic signs were used to form their coacervate.


 Please wait while we load your content...
Please wait while we load your content...