Facile synthetic approach towards vasorelaxant active 4-hydroxyquinazoline-4-carboxamides†
Abstract
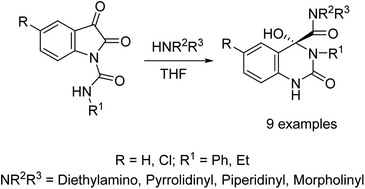
A Facile synthetic approach is reported towards 4-hydroxyquinazoline-4-carboxamides 13a–i through ring expansion of 2,3-dioxoindoline-1-carboxamides 10a–c during secondary amine 11a–d nucleophilic reaction. Single crystal X-ray studies of 10c and 13d support the structures. Some of the synthesized quinazolinecarboxamides 13 show promising vasorelaxant properties with potency higher than that of Doxazosin through the pre-contracted (norepinephrine hydrochloride) rat aorta standard bioassay. Good molecular models (2D-QSAR, pharmacophore) describe the biological observations.


 Please wait while we load your content...
Please wait while we load your content...