Heavy metal removal from aqueous systems using hydroxyapatite nanocrystals derived from clam shells
Abstract
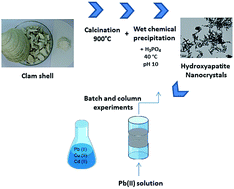
Hydroxyapatite (HA) was synthesized by wet chemical precipitation, using clam shell (CS) waste as feedstock. SEM and TEM observation of the produced hydroxyapatite revealed the presence of rod-shaped nanocrystals, while XRD and EDS analyses confirmed the characteristic patterns of hydroxyapatite molecules. This material was subsequently employed as a sorbent for heavy metal removal from aqueous solutions, both in batch and column equilibrium procedures. In batch studies, higher sorption efficiencies were obtained at pH 5, with the highest adsorption capacities of 265, 64, and 55 mg g−1 for Pb(II), Cd(II), and Cu(II), respectively. In addition, an adsorption capacity of 42.5 mg g−1 was determined using a CS-HA packed bed column fed with a solution of Pb(II). Finally, the breakthrough curve was fitted with Thomas model in order to predict column behavior and scaling up.
- This article is part of the themed collection: Celebrating Latin American Talent in Chemistry


 Please wait while we load your content...
Please wait while we load your content...