Efficient solid-state emission and reversible mechanofluorochromism of a tetraphenylethene-pyrene-based β-diketonate boron complex†
Abstract
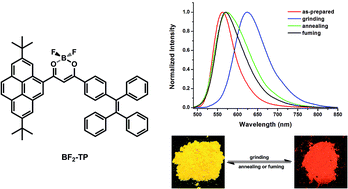
A new twisted donor–acceptor (D–A) dye (BF2-TP) that was composed of tetraphenylethene and pyrene connected with a β-diketonate boron moiety has been synthesized and characterized. Such a dye showed unique intramolecular charge transfer (ICT) features, which were evidenced by spectral analysis and theoretical calculations. More importantly, BF2-TP solid samples exhibited an obvious mechanofluorochromic (MFC) behavior. Upon grinding with a spatula, the as-prepared powder sample illustrated a remarkable red shift of 62 nm, with considerable color contrast from yellow (562 nm) to orange red (624 nm). Its fluorescence color can be reversibly switched by repeating both the grinding–fuming and grinding–annealing processes. The mechanochromism is attributed to the phase transformation between amorphous and crystalline states. The results obtained would be helpful for designing novel MFC materials.
- This article is part of the themed collection: Editors’ collection: Fluorescent Sensors


 Please wait while we load your content...
Please wait while we load your content...