Cobalt, nickel and copper complexes with glycinamide: structural insights and magnetic properties†
Abstract
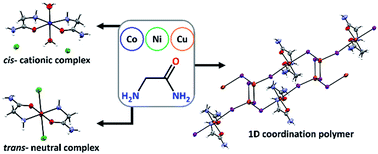
Ten new compounds of Co, Ni and Cu with glycinamide (HL = glycinamide): [Co(H2O)2(HL)2]Cl2 (1a), [Co(H2O)2(HL)2]Br1.06Cl0.94 (1b), [Co(H2O)2(HL)2]I2 (1c), [Ni(H2O)2(HL)2]Cl2 (2a), [Ni(H2O)2(HL)2]Br0.94Cl1.06 (2b), [Ni(H2O)2(HL)2]I2 (low and room temperature polymorph, 2cLT and 2cRT), [CuCl2(HL)2] (3a), [CuBr1.3Cl0.7(HL)2] (3b) and {[Cu(HL)2]2[Cu2I6]}n (3c), as well as glycinamide hydroiodide (H2LI) and a new polymorph of glycinamide hydrochloride (β-H2LCl) were prepared and characterized by single-crystal X-ray diffraction, infrared spectroscopy, thermal analysis (TG/DTA) and ESR spectroscopy. 1a, 1b, 2a and 2b are isostructural, as well as 1c and 2cRT, while the Cu compounds (3a–c) have entirely different molecular structures. All investigated compounds are mononuclear with exception of the 1D coordination polymer 3c. Compound 3c contains copper ions in the mixed oxidation state Cu(I) and Cu(II) with interesting magnetic properties. Paramagnetic behaviour was found in 1a, 1b, 3a and 3b. Temperature induced polymorphic transformation was observed in 2c. Compounds 1a and 3a showed moderate antiproliferative activity and selectivity toward the human breast tumor cell line MCF-7.


 Please wait while we load your content...
Please wait while we load your content...