Facile thermochemical conversion of FeOOH nanorods to ZnFe2O4 nanorods for high-rate lithium storage
Abstract
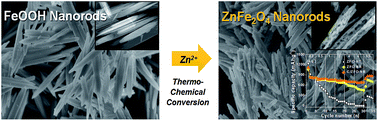
We successfully prepared ZnFe2O4 nanorods (ZFO-NRs) by a simple thermochemical reaction of FeOOH nanorods with Zn(NO3)2 to use as an anode material in lithium-ion batteries. The FeOOH nanorod shape was well maintained after conversion into ZFO-NR with the formation of porous structures. The nanorod structure and porous morphology facilitate Li+ transport, improve the reaction rates owing to the larger contact area with the electrolyte, and reduce the mechanical stress during lithiation/delithiation. The ZFO-NR electrode exhibited a reversible capacity of 725 mA h g−1 at 1 A g−1 and maintained a capacity of 668 mA h g−1 at 2 A g−1; these capacities are much higher and more stable than those of ZFO nanoparticles prepared by a hydrothermal method (ZFO-HT) (216 and 117 mA h g−1 at 1 and 2 A g−1, respectively). Although ZFO-NRs exhibited high, stable capacities at moderate current densities for charging and discharging, the capacity rapidly decreased under fast charging/discharging conditions (>4 A g−1). However, carbonized ZFO-NR (C/ZFO-NR) exhibited an improved reversible capacity and rate capability resulting from an increased conductivity compared with ZFO-NRs. The specific capacity of C/ZFO-NRs at 1 A g−1 was 765 mA h g−1; notably, a capacity of 680 mA h g−1 was maintained at 6 A g−1.


 Please wait while we load your content...
Please wait while we load your content...