Tuning the Biginelli reaction mechanism by the ionic liquid effect: the combined role of supported heteropolyacid derivatives and acidic strength†
Abstract
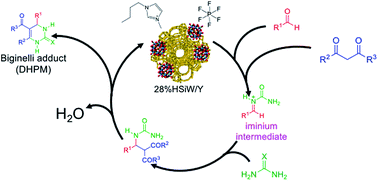
Herein, a combination of heteropolyacids and ionic liquids as a catalytic system was studied for the Biginelli multicomponent reaction; the positive ionic liquid effect associated with the acidic strength of zeolite-supported heteropolyacids made this combination an efficient catalytic system for the multicomponent synthesis of 3,4-dihydropyrimidin-2(1H)-one/thione derivatives. The acidic strength effect was evaluated, and a range was determined in which the reaction provided better results. The mechanism of the reaction was also investigated in the presence and absence of ionic liquids, and two features of paramount importance were revealed: the mechanism could be tuned to proceed through only one reaction path among three possibilities and the kinetics of the reaction was significantly faster in the presence of an ionic liquid.
- This article is part of the themed collections: Celebrating Latin American Talent in Chemistry and How does it work? – a collection on mechanistic studies


 Please wait while we load your content...
Please wait while we load your content...